Jin-Moo Lee, MD, PhD, head of the WashU Medicine Department of Neurology, and Eric Leuthardt, MD, MBA, vice chair of innovation in the Department of Neurosurgery, have been selected as recipients of the prestigious Falk Catalyst Award.
Each year, select institutions are invited to submit up to two applications to the Dr. Ralph and Marian Falk Medical Research Trust – Catalyst Award Program. Funded by the Dr. Ralph and Marian Falk Medical Research Trust, Bank of America, Private Bank, the Catalyst Award program provides up to $350,000 in seed funding, supporting high-risk, high-reward research projects that address significant therapeutic challenges. Applications with the potential to create novel pathways for innovative approaches in disease treatment and cures are selected through a rigorous review process by Health Resources in Action (HRiA), a nonprofit consultancy that makes funding decisions on behalf of the Falk Trust.
The Catalyst Research Award is intended to facilitate translational research and enable implementation into clinical practice in the near term. Successful projects may choose to transition into commercial development via the trust’s larger transformational research award. Jeffrey R. Millman, PhD, professor of medicine and of biomedical engineering at WashU Medicine, was also selected as a 2025 Catalyst Award recipient.
The Lee lab at WashU Medicine will use the secured funding to continue their work on a project that aims to reduce brain inflammation and injury after large vessel occlusion (LVO) stroke using vagus nerve stimulation. Lee is the neurologist-in-chief at Barnes-Jewish Hospital and is an internationally renowned expert in stroke and vascular neurology. His work spans the full spectrum from basic investigations using cell and animal models to clinical studies using multimodal MRI and human genomics. Specifically in the field of stroke, Lee’s research and clinical care range from acute ischemic brain injury to long-term recovery.

Titled “Neuromodulation Using Vagus Nerve Stimulation Following Ischemic Stroke as Therapeutic Adjunct (NUVISTA) 2,” the study plans to enroll 65 acute stroke patients with LVO to undergo vagus nerve stimulation using a non-invasive device. The Lee lab will use biomarkers in the blood to determine whether the intervention is able to reduce brain inflammation in LVO stroke patients during the acute recovery stage, with the ultimate goal of reducing the likelihood of long-term disability in those patients.
The World Stroke Organization’s 2025 Global Stroke Fact Sheet notes that “among non-communicable disorders, stroke remains the second leading cause of death and the third leading cause of death and disability” worldwide. Even when individuals survive the initial attack, stroke leads to permanent disabilities in up to 60% of cases. This is in part because stroke triggers widespread inflammation that worsens brain injury. Multiple clinical trials have tried to reduce neuroinflammation in stroke patients using drugs that target individual inflammatory pathways. However, virtually all have failed, demonstrating a need for novel approaches that more broadly suppress inflammation.
Lee and Leuthardt’s NUVISTA 2 trial uses an innovative, non-invasive device to stimulate the vagus nerve and directly modulate inflammatory cells in the spleen through neuro-immune synapses. The small, over-ear devices for trans-auricular vagus nerve stimulation (taVNS) were developed by Leuthardt, the Shi H. Huang Professor of Neurological Surgery at WashU Medicine, and his company, Aurenar. Aurenar is pursuing the FDA’s De Novo approval pathway for its device. Leuthardt serves as chief of the Division of Neurotechnology in the Taylor Family Department of Neurosurgery at WashU Medicine and has an extensive background in translational neuromodulation technologies, with numerous issued or pending patents around the technology of taVNS and its applications to stroke.
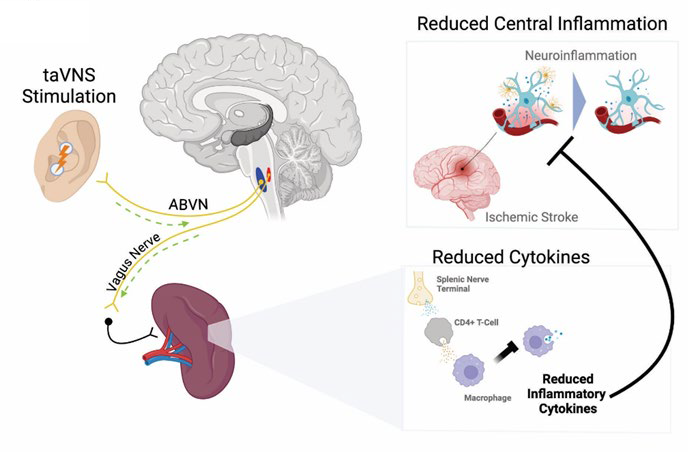
One of the key strengths of taVNS devices is the lack of known side effects. “Vagus nerve stimulation devices have already shown promise in the pilot stage of our study and, if proven to be effective, could be used to treat 800,000 stroke patients per year in the U.S. because of how easy they are to use,” said Lee, who is the Andrew B. and Gretchen P. Jones Professor of Neurology at WashU Medicine.
The study will be conducted by a multidisciplinary team that combines expertise in neurology, neurosurgery, emergency medicine, neurogenomics and clinical trial operations. Lee and Leuthardt will oversee all aspects of the study in collaboration with Opeolu Adeoye, MD, MS, Carlos Cruchaga, PhD, and Osvaldo Laurido-Soto, MD.
